Imitrex SC
Imitrex SC
Imitrex SC contains the active substance sumatriptan .
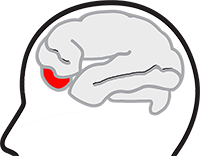
It is a serotonin receptor agonist (5-HT 1B / 1D) given, among others, in the treatment of cluster headache in adults.
The subcutaneous injection of Imitrex is one of the two main attack treatments. It is not a background therapy.
An ECG (electrocardiogram) is necessary before this treatment is proposed. In fact, Sumatriptan is to be avoided by people at cardiovascular risk or with high blood pressure (> 15/9).
Remark
The Imitrex SC 6 mg injectable solution is, in the vast majority of cases, extremely effective within a few minutes.
However, in rare cases the injection is ineffective against the attacks. Patients should discuss this with their neurologist and avoid overdoses!
For others, and many overlook this, injecting a partial dose is plenty and often causes fewer side effects.
Talk about this to your neurologist.
General Description
| Product Name: | IMITREX SC 6 MG 2 SER INJ |
| CNK code: | 307595 |
| Category : | Reimbursed in category B |
| Contents : | 2 injectable vials |
| Lab : | GLAXOSMITHKLINE PHARMACEUTICALS |
Imitrex SC 6 mg solution for injection contains 6 mg of sumatriptan in the form of sumatriptan succinate in an isotonic solution with a total volume of 0.5 ml.
Excipient: Sodium chloride – water for injections.
Different Names
Sumatriptan for subcutaneous injection has different names depending on the country where it is available:
- Imitrex SC (Belgium, …)
- Imiject SC (France, …)
- Imigran SC (UK, …)
- Imigrane SC (France, …)
All are manufactured by GSK, it is exactly the same product.
Generic versions of the drug exist but not in Belgium (data from 2019):
- Sumatriptan SUN (SUN Pharma) (France, Netherlands, …)
- Sumatriptan-Hormosan (Hormosan Pharma) (Germany)
Dangers of Sumatriptan Abuse
The first danger is of a cardiovascular nature: significant and repeated vasoconstriction can have severe consequences.
Do not exceed two injections per 24 hours and allow at least one hour to pass between two complete injections.
Sumatriptan should not be given to patients with a history of myocardial infarction or ischemic heart disease, coronary vasospasm (Variant angina), peripheral vascular disease or to patients with symptoms of ischemic heart disease or any signs compatible with ischemic heart disease.
The second danger is the risk of medication-induced headaches which will worsen the patient’s condition!
See Imitrex Studies
Overview of the Instructions
Action
The subcutaneous injection (SC) of Imitrex treats the cluster headache attack in a few minutes.
Dosage
Please note: Imitrex SC should not be used as a background therapy !
The recommended dose is one injection of 6 mg subcutaneously during each attack but the maximum dose in 24 hours is two injections of 6mg (12mg) with a minimum time interval of one hour between the two injections.
This clearly poses a problem for patients who suffer from more than two attacks per day.
It is advisable to give the injection as soon as possible, at the onset of the attack.
Adverse Effects
There are more or less pronounced side effects depending on the individual:
- Hot flushes
- Dizziness
- Chest tightness
These troubles generally last for a short period of time.
Contraindications
- As with all medicines, do not use Imitrex in case of hypersensitivity to the active substance (here sumatriptan) or to the excipient (here sodium chloride).
- If you have had a myocardial infarction or if you suffer from ischemic cardiomyopathy, coronary vasospasm, or peripheral vascular disease, consult a cardiologist.
- If you have had a myocardial infarction or if you suffer from ischemic cardiomyopathy, coronary vasospasm, or peripheral vascular disease, consult a cardiologist.
- Imitrex is also contraindicated for patients with a history of stroke or transient ischemic attack (TIA).
- Also, and it’s less known, talk to your doctor if you have severe hepatic impairment.
- Imitrex is, more commonly, to be avoided by patients suffering from moderate or severe hypertension , or mild hypertension which is uncontrolled.
- Finally, the co-administration of ergotamine or ergotamine derivatives (e.g. Cafergot) is strongly discouraged.
Studies
When doing a search on Pubmed for “cluster headache” and “sumatriptan” we got 264 results !
This is not to say that all of these studies concerning the sumatriptan injection (Imitrex SC) are double-blind, placebo-controlled studies.
Below you will find a link to studies of interest.